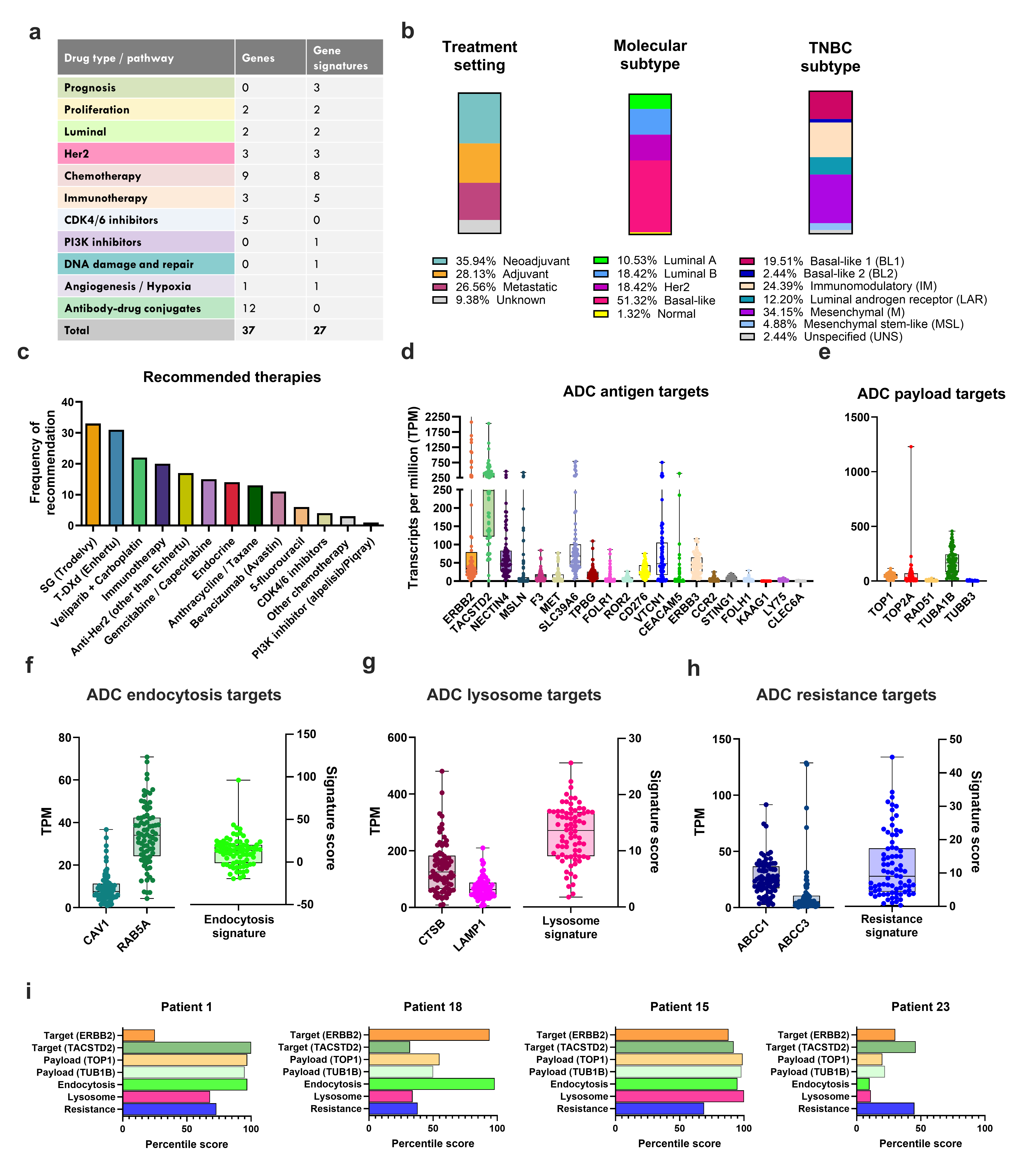
About MultiplexDX
MultiplexDX is one of the most innovative biotech companies, headquartered in Bratislava, Slovakia, created to bring its revolutionary technologies to the market of personalized molecular diagnostics. It was founded in 2016 by Dr. Pavol Cekan who has extensive experience in the fields of biotechnology research in EU and US ecosystems. The company operates in the Science Park of Comenius University and has a dedicated international team of 30 people, complemented by the expertise of our top-level advisory board consisting of world-renowned 2 scientific and 2 business advisors. MDX has achieved remarkable success since its foundation. The company has received numerous awards. It was also recognized as one of the 2017 New Europe 100 Challengers by the Financial Times, Google, and Visegrad Fund. MDX‘s journey includes participation in top-tier accelerators such as the Health Hub Vienna Acceleration Program for biotech start-ups and membership in the Oslo Cancer Cluster. In 2019, MDX became the first SK company in history to be awarded the prestigious EIC Accelerator in which it successfully validated its innovative technology in a large-scale retrospective validation on 1,080 breast tumors. In 2022, MDX achieved a significant milestone by ranking 1st in the TOP3 of the Deloitte Fast 50 CEE. In 2023, the company diagnosed the first 26 patients with breast cancer and signed 4 MoUs with oncology institutes in SK and others in process. In 2023 MDX was chosen, among only 47 EU companies and only 10 SMEs, to be part of the Global Gateway Business Advisory Group to assist the European Commission.